DISCUSSION
I. MANUALLY ISOLATED POLYTENE CHROMOSOMES AND NUCLEAR ENVELOPES
1. Technical
For EM inspection of biological material, mica has been used earlier for viruses (Horne, Ronchetti and Hobart, 1975), nucleic acids (Portman and Koller, 1976) and for high resolution protein studies (for the EM use of mica see Willison and Rowe, 1980). However, to our knowledge we were the first to use it as a support of high affinity for the extraction and enzyme digestion studies of whole organelles such as nuclei, NE and delicate material like chromosomes, for which we found it to be perfectly suited (Engelhardt and Plagens, 1980a, b).
It could be argued that enzymes may not work properly when the material has been attached. Light microscopic monitoring, however, proved that digestion did proceed. After DNase digestion, for example, no DNA strands have been found, unlike after control treatments without DNase or using denatured DNase. It could also be argued that remnants of nucleic acids can stay protected by proteins, but this applies to bulk digestion as well, in which case no more than 0.1-1% of DNA and 1% of RNA are left in the matrix (Berezney and Coffey, 1974, 1976), the scaffold (Adolph et al., 1977), and the NE (Aaronson and Blobel, 1975; yet with other methods, higher amounts have been reported, see Agutter and Richardson, 1980.)
Extractions and successive digestions in bulk are probably hampered by clumping and aggregation of the material (because of the many centrifugations and dispersions) more than with the mica flat-mounting method, although also here local microaggregations could occur. Yet, it is always possible to find thinly spread peripheral areas on the mica for examination. A further advantage of the method is that the material is continuously immersed in solution, not exposed to air as with the standard spreading techniques. Standard spreadings picked from the surface of water are in danger of oxidization and denaturation (cf. Solari, 1972), which may impair digestions (Gould, Callan and Thomas, 1976). More importantly, spreading methods on bulk-extracted and treated chromosomes for EM (cf. Paulson and Laemmli, 1977; Mullinger and Johnson, 1980) may ruin the delicate foldings of DNA, leading to destruction and loss of structural components.
2. The nature of the scaffolds: globular vs. fibrous elements
Using these new methods the "scaffold" of the chromosomes could be visualized and its structure compared to that of similarly treated NEs. Polytene chromosomes are lateral bundles of extended interphase chromosomes in which the band regions are considered to represent orderly assemblies of chromomeres. From the results it can be concluded that there exists indeed an underlying residual core behind chromomeres which is resistant to high salt (2 M solution), to detergent and nucleases. The core is made up of unit elements morphologically indistinguishable from those of the residual NE or more precisely the annular subunits of the NPC. This becomes apparent especially after hyaluronidase digestion.
These unit elements are the main structural component of all parts of the scaffold that could be examined and this is confirmed by hyaluronidase treatment. They occur in different assemblies: linear or ring-form oligomers. They may stay in smaller or bigger aggregates, yet they are the only structural elements exposed in stereo pairs.
The unit elements themselves are somewhat problematic in their fine structure. Morphologically they seem to have a dual appearance: globular (globosomes) or ring-like (cyclosomes) (Engelhardt and Plagens, 1980a), both with a diameter of 25-30 nm (total range 20-45 nm). It is not yet clear, whether these alternative structures depend on treatment, functional state or just the EM technique (carbon evaporation, Pt shadowing). It has to be emphasized regarding the NPC subunits that also their dual nature has been earlier described in thin section studies (Engelhardt and Pusa, 1972; Maul, 1977) and in whole mounts, with negative staining (Harris, 1978; Harris and Marshall, 1981).
The nuclear protein matrix and the chromosome scaffold have been described and verified in numerous previous studies using different methods and a role has been claimed for them in many nuclear and chromosomal functions (for recent reviews see: Nelson, Pienta, Barrack, and Coffey, 1986; Newport and Forbes, 1987; Gasser and Laemmli, 1987; Paulson, 1988; Earnshaw and Heck, 1988). Sometime ago their existence was under dispute (Okada and Comings, 1980; Hadlaczky, Sumner and Ross, 1981; for more recent discussion on this matter see Harris, 1985, and Paulson, 1988). As yet, their precise structure is not known. In this study, a morphological and comparative approach has been adopted and a new and more detailed description and characterization has been possible.
Now that the unit elements, globosomes-cyclosomes, have been detected, it is somewhat contradictory that the matrix and scaffold have generally been regarded as composed of a sponge-like or fibrous network (Berezney, 1980; reviewed by Agutter and Richardson, 1980; Earnshaw and Heck, 1988). Such conclusions have been made either because they were based on rough approximations at the gross level, or because the structures are resolved only with negative staining (matrixin, Comings and Okada, 1976), since after all extractions and digestions no real, fibrous structures were found in or behind such networks. The apparent coarse fibres or "cables" (Earnshaw and Heck, 1988) are made up of the unit elements, globosomes-cyclosomes.
Hyaluronidase was especially valuable because it dispersed and exposed the whole composite structure into component elements. This need not imply that there had been fibres before hyaluronidase treatment. Only the general appearance of the scaffold is sponge-like or roughly fibrous, especially after RNase treatment (pH 5.2), which seems to cause considerable contraction in the sponge-like network. Certain fibres which look like "contours" and single rings (both in carbon material) are dense peripheries of compact material. All this may create the impression of fibrous structures.
It may seem strange that such globular elements should have previously passed unnoticed. Taking a close look at only some published pictures of the matrix and scaffolds (Paulson and Laemmli, 1977; Okada and Comings, 1979; Comings and Okada, 1980), unmistakable globular structures can really be seen, e.g. in the periphery of the scaffold networks. These globules were ignored, maybe because the remaining structures were too compact for interpretation.
Later, globular matrix units have been isolated and described as RNP-containing particles (Berezney, 1980). Peripheral globular units have been noticed in some scaffolds but for some reason they were considered "unextracted nucleosomes" (Okada and Comings, 1979). Already, some 20 years ago spheroidal particles of the nonhistones or nuclear residual proteins, after Pt shadowing, were described (Patel, Patel, Wang and Zobel, 1968).
As the matrix of certain cell types has been reported as fibrous "matrixin" (Comings and Okada, 1976; Berezney, 1980), the existence of fibres cannot be ruled out only because they cannot be found in the scaffolds studied.
If the sponge-like appearance of the matrix-scaffold is only an apparent gross view, could there be an underlying fibrous structure on which the particles are aligned? Over some interband areas, the scaffold is stretched (from two adjacent bands?) into apparent "cables," which could descriptively be called a typical sponge-like network of scaffold fibres (Fig. 23), but even in such places, studied in stereo at higher magnifications (Figs. 27, 30), no hidden real fibres could be found. There are only globular elements in roughly linear arrays with clear gaps between the globules. Occasionally, however, where the gaps are wider, something like very fine fibrous strands can be vaguely seen (Fig. 30), suggesting local remnants, which may result from incomplete digestion of nucleic acids (?) effectively protected by these particles. This particularly concerns scaffolds that were also digested with hyaluronidase.
Without hyaluronidase digestion, the sponge-like scaffold is much too aggregated and amorphous to allow a search for possible fibres. This points to a hyaluronidase-sensitive filling and covering material which is converted or digested by the enzyme, so that the globular units are more exposed. Even when the units are deformed in aggregates, their contours are very clear (Figs. 27-30). No real, fibrous structures were exposed in less aggregated areas before hyaluronidase treatment, showing that all existing core fibres are digestible with the nucleases, specially DNase.
A possible structural role of RNA could be demonstrated by the use of RNase (at pH 5.2), which produced a typical condensing effect on bands. The sponge-type network of the whole scaffold is pronounced (compare Figs. 2, 4, 5, 23, 27, and 3, 24, 28). Yet, because hyaluronidase treatment does not show typical fibres (without RNase), the RNase effect is probably an internal change to general compactness. This is understandable if RNase has a converting effect on the unit particles themselves which have a dual appearance. But, this difference can only be seen in carbon-evaporated material (without Pt shadowing) (Fig. 32). Pt shadowing may, because of its high contrast, hide these minor inner differences.
The condensing effect of RNase on chromatin generally has also been found in interphase nuclei (Derenzini, Pession-Brizzi and Novello, 1980) and metaphase chromosomes in which Giemsa bands were produced (Rønne, 1977). Whatever the RNase effect is, it can hardly be a simple reversal of "puffing." It occurs in every band, which shows a true structural role for some type of intrinsic RNA. The reason behind it could be that RNA sequences could be involved in linking DNA to scaffold elements, so that RNA digestion released the DNA, thereby producing a contraction of the scaffold by cohesive forces.
The effects of these extractions and digestions may be more complicated than has been assumed, because the treatments may cause reorganization of substances and hamper digestion. Besides, a new interesting claim has recently been made that several commercial preparations of "highly purified" RNase A contain an "occult" protease activity (Fischer, 1988). If this is true, some of the peculiar RNase effects described may get a reasonable explanation.
Altogether, there is no direct proof of real scaffold fibres, although the scaffold-forming elements often take roughly fibrous arrangements, maybe along remnant DNA which stays resistant to complete digestion. From the smallest heaps of few particles to bigger and bigger aggregates, there was in this material no other morphological differentiation than the clusters of globules, which could be partly digested by the "occult" protease activity of the RNase A and get "sticky". Artificial fibrils would thus appear between the globules under streching and washing (cf. Fig. 30).
It cannot be ruled out that there is, after all, a proteinaceous fibre network onto which the globular elements are normally ordered in chromosomes. Rearrangements in this network could be caused by experimental treatments and no doubt during normal chromosomal functions.
Nor can a fibre-to-globule conversion (treatment-dependent?) be excluded. Some evidence exists that the globules themselves can be seen, in stereo-inspection, to unravel into vague, filamentous (under 1.5 nm in diameter) strands (Engelhardt and Plagens, 1984). This was made possible by using a high resolution mica-based negative staining technique (Horne, Ronchetti and Hobart, 1975).
In material treated with salt and hyaluronidase (omitting RNase and DNase), the folding of DNA could be followed mostly round the oligomeric assemblies (rings and rods). Monomeric units did not appear, as an overwhelming cascade, if DNase was not involved, either before or after hyaluronidase (with or without RNase involved). This is evidence that DNA is also involved as an integrating factor of the oligomeric forms.
Other integrating factors are indicated because various sizes of rings including linear forms, besides the cascades of monomers in the neighbourhood, are still observed in salt, RNase, DNase and hyaluronidase-treated chromosomes. The mode and degree of the assembly, whether by specific linkers or just juxtaposition, are not known. In ring forms the assembly seems much tighter, as the subunits are less distinct, their borders smoother and gaps less evident than in many linear forms. A gradual transformation from linear forms (mostly of 5-7 particles) and half-arches to almost closed rings proves (Fig. 33), however, that all these are made up of the same units.
Naturally, micro-heterogeneity in composition and function could exist, especially for the NE and the band scaffold. This could also be involved in the dynamic changes such as attachment-detachment from the NE.
3. Hyaluronidase digestibility of the scaffold
With a one-hour hyaluronidase digestion (before or after nucleases), which produced cascades of monomer units from band and NE scaffolds, most band regions and the NE stayed aggregated, but unit elements were clearly exposed on surfaces. The resistance to complete disintegration may reflect a lack of further hyaluronidase-sensitive material, or steric hindrance resulting from aggregation. Copper could act as a natural inhibitor of hyaluronidase: chromatin contains a Cu-metalloprotein (Bryan, Vizard, Beary, Labiche and Hardy, 1981; Sigee and Kearns, 1981) and Cu-protein interactions have been strongly indicated in the scaffold (Lewis and Laemmli, 1982).
No structural basis for hyaluronidase sensitivity could be determined, but clearly one of the factors that aggregate scaffold-forming units seemed to be a hyaluronidase-sensitive material, because the described globular subunits were released and exposed by treatment with this enzyme.
It can be difficult to demonstrate the fibrous nature of the lamina of the pore-lamina fraction (Agutter and Richardson, 1980). According to Richardson, detergent destroys the visualization of lamina fibres, and the preparation technique, which always included Triton X-100 and dehydration with methanol, may therefore, have caused further agglutination into a more amorphous and unresolvable form.
There are clear indications that the lamina, lining the inner NE (fibrous lamina), contains carbohydrates, as it stained with ruthenium red (Kalifat and Dupuy-Coin, 1970) and studies show that the lamina does truly incorporate glucosamine (Mancini, Heywood and Hodge, l973). According to the present results, this same material could be involved in the matrix-scaffold as the hyaluronidase-sensitive aggregating material.
Immunotechniques with lamina-specific antibodies suggest, however, that lamina polypeptides occur only at the periphery of the nucleus, not inside (Ely, D'Arcy and Jost, 1978; Gerace, Blum and Blobel, 1978; Krohne, Franke, Ely, D'Arcy and Jost, 1978; Stick and Hausen, 1980; Jost and Johnson, 1981, for DNA-bound proteins of similar molecular weight, see also Werner, Zimmermann, Rauterberg, and Spalinger, 1981). Neither have nuclear lamins been proven to be involved in metaphase chromosome structure, as they seem to have diffused into the cytoplasm in metaphase (refs. above).
Polytene chromosomes may be a special case, as they are interphase chromosomes, although amplified and highly packed into lateral bundles. Here, lamins could be present also inside the nucleus, but only a staining of the peripheral rim has been seen in cryosections (reviewed by Fischer, 1988). No studies have yet been done with lamina antibodies on the scaffold of polytene chromosomes, and indeed the presence of DNA could be a hindrance to the antibodies.
Immunochemical labelling can be altered by many factors besides steric hindrance and protein denaturation. A small change in terminal saccharides may be decisive (Sharon, 1980). Such changes could regulate the attachment-detachment changes of chromosomes and the NE.
Also there are some observations (Engelhardt and Trepte, unpublished) which show that lamina-like material could be involved. When digesting polytene chromosomes with nucleases, it can be seen in phase contrast, that some material still keeps the bands and the whole chromosome longitudinally together in the buffer solution, although attached at only some parts like a telomere end to the mica support. This material cannot be DNA, as DAPI staining does not produce any detectable fluorescence.
Futhermore, in EM thin sections of salivary gland nuclei of Chironomus (Engelhardt and Trepte, unpublished), a thin continuous peripheral membranous layer can be seen around the chromosomes (sometimes also in internal compartments of the chromosomes); it is insoluble in detergent (Triton X-100), 2 M salt solution and undigestable by nucleases. This could represent some manifestation of lamina or lamina-like material, as the same material is seen as the lamina of the NE in the same nuclei. This material can be too delicate to detect in whole mounts with the mica technique, as it probably collapses in air-drying to an unrecognizable form. This membranous lamina-like material is seen partly destroyed, in thin sections, in hyaluronidase-treated preparations. This supports the results from the whole mount mica technique, that there is a hyaluronidase-sensitive filling and covering layer in the band scaffolds.
The successful application of hyaluronidase to reveal the structure of the scaffold was based on the suggestion that carbohydrates are important integrating components of the eukaryotic chromosomes, as recognition sites for their attachment to the NE and for the specificity of pairing. In our earlier studies we could observe that under treatment with hyaluronidase the NE and chromosomes lose their affinity for ruthenium red stain, the nuclear membranes undulate and separate and the synaptonemal complexes disappeared (Engelhardt and Pusa, 1972a, b).
We could confirm the disintegration of the synaptonemal complexes in detail as they fell apart into unit elements which were morphologically similar to scaffold units (Engelhardt, Plagens and Pusa, 1980). This hyaluronidase-sensitive material has not been identified. Secondary effects of hyaluronidase cannot be completely excluded.
4. Carbohydrates in chromosomes, nuclei and NE
There have been few data available on glycosaminoglycans (GAG) in chromosomes, nuclei and NE generally. At the light microscopic level hyaluronidase has been used earlier to get a better separation of chromosomes (Marshall, Wood and Bierman, 1961) and to distinguish subchromatids (Iino, 1971; Molina, 1974). There have also been histochemical demonstrations of polysaccharides associated with chromosomes including polytene chromosomes (Ohnishi, Yamamoto and Terayama, 1973).
It can also be confirmed that manually isolated whole nuclei of Chironomus salivary gland cells, polytene chromosomes and NEs show distinct staining with the ruthenium red methods. The dark carbohydrate-specific staining is changed after hyaluronidase (Engelhardt and Trepte, unpublished).
Besides the scanty histochemical evidence for carbohydrate-containing substances in the nuclei, chromosomes and NEs, scattered in the literature (see Engelhardt and Pusa, 1972a for earlier references), more direct evidence has been accumulated (for reviews see Stoddart, 1979; Stein, Roberts, Stein and Davis, 1981).
Many nonhistone proteins incorporate tritium-labeled glucosamine (Stein, Roberts, Davis, Head, Stein, Thrall, Van Veen and Welch, 1975). GAG has been successfully extracted from rat brain cell nuclei (Margolis, Crockett, Kiang and Margolis, 1976), HeLa nuclei (Bhavanandan and Davidson, 1975), and regenerating rat liver nuclei (Furukawa and Terayama, 1979), and most interestingly, the latter also show significant levels of hyaluronic acid.
According to the analysis of NE proteins by Sieber-Blum and Burger (1977), of the 47 proteins from the NE, 9 contain aminosugars. The presence of carbohydrates in the NE is by no means excluded in highly purified NE preparations (Scheer, Kartenbeck, Trendelenburg, Stadler and Franke, 1976; Franke, Keenan, Stadler, Genz, Jarasch and Kartenbeck, 1976; more references on NE-bound carbohydrates in the latter). There are also demonstrations of high-molecular weight glycoproteins in some NE fractions (Monneron and d'Alayer, 1978).
Certain lectins has been shown to bind to the NE (Nicolson, Lacorbiere and Delmonte, 1972; Virtanen and Wartiovaara, 1976; Feldherr, Richmond and Noonan, 1977), chromatin (Rizzo and Bustin, 1977), synaptonemal complexes (Engelhardt, Virtanen and Pusa, 1977) and polytene chromosomes (Kurth, Bustin and Moudrianakis, 1979; Engelhardt and Trepte, unpublished).
Glucosamine and sialic acid can be incorporated into the nuclear protein matrix (Wunderlich, Berezney and Kleinig, 1976). According to direct analyses, the nuclear matrix from rat liver cells contain 5.5% neutral sugars including glucose, mannose, galactose and an unidentified sugar (Berezney and Coffey, 1977); also the matrix of Tetrahymena was found to contain 0.9% carbohydrate, i.e. glucose, mannose and an unidentified sugar in the ratio of 1 : 5.4 : 5.7 (Wunderlich and Herlan, 1977).
Furthermore, a high-molecular weight glycoprotein was recently demonstrated in the nuclear matrix and NPC-lamina of several animal species (Fisher, Berrios and Blobel, 1982; Filson, Lewis, Blobel and Fisher, 1985; see also a recent review by Fischer, 1988). A very similar high-molecular weight glycoprotein (gp190) was identified as a major concanavalin A-binding NPC-lamina protein insoluble in the nonionic detergent TX-100, by Gerace, Ottaviano, and Kondor-Koch (1982). With antibodies to this protein, it could be shown that it was specifically situated only at the cytoplasmic side of the NPC with immunoferritin electron microscopy. No labeling could be detected without detergent thus demonstrating an integral membrane location. It was proposed that this structural protein linked the NPC within the NE, where the Con A-binding moiety was situated in the perinuclear space, by analogy with other transmembrane glycoproteins.
It was a breakthrough of recent years that some new unusual NPC-specific glycoproteins could be identified, namely with the lectin WGA (wheat germ agglutinin). They contain single additions of O-linked N-acetylglucosamine residues situated either at the cytoplasmic or nucleoplasmic side of the pore complex (Davis and Blobel, 1986, 1987; Snow, Senior and Gerace, 1987; Holt, Snow, Senior, Haltiwanger, Gerace and Hart, 1987; Park, D´Onofrio, Willingham and Hanover, 1987; Hanover, Cohen, Willingham and Park, 1987).
Earlier it had been observed that WGA binding stopped the efflux of RNA in nuclei (Baglia and Maul, 1983). It was also recently established that WGA stops protein transport. Rhodamine-labeled nucleoplasmin transport into the nuclei was inhibited by WGA (Finley, Newmeyer, Price, and Forbes, 1987). Ferritin-labeled WGA could be very precisely localized by electron microscopy to the cytoplasmic face of the NPC (about 15 copies of WGA per pore). Among the lectins tested, only WGA had this effect.
N-linked glycans are known to be the common linkage of glycoproteins, especially the proteins of the membranes, where the sugar residues are situated only on the external sides. In contrast, O-linked glycans are exposed on both the cytoplasmic and the nucleoplasmic compartments, presumable synthesized by different routes and mechanisms. It was unexpected that this type of glycoprotein is enriched in the NPC and nucleoplasm.
As early as 1972 histochemical evidence had been provided that glycosylated compounds must be present in subunits of NPC, fibrous lamina, cyclomeres, synaptonemal complex and in other cytoplasmic organelles. This was done using ruthenium red staining methods, which were developed to allow the penetration of stain to nuclei to enhance carbohydrate-containing substances (Engelhardt and Pusa,1972a, b). Similar methods were developed for alcian blue (Engelhardt, unpublished) and lectins to stain isolated meiotic nuclei (Engelhardt, Virtanen and Pusa, 1977) and polytene chromosomes (Engelhardt and Trepte, unpublished).
The specificity of these staining methods could be demonstrated by: (i) hyaluronidase incubation and (ii) using known membrane glycoproteins such as rhodopsin as model substrates to localize the carbohydrate moieties in the disc membranes of retinal rod outer segments (Engelhardt and Storteir, 1975; Engelhardt, 1979). The carbohydrate-containing part of the rhodopsin could be localized precisely, and in high resolution, in the pericisternal side (the lumen) of the disc membranes, whereas the protein part was in the cytoplasmic side, demonstrating that the rhodopsin is a typical transmembrane glycoprotein (see the review by Corless, 1980: in which some of our plates have been printed and discussed in detail).
5. Proteins of the cytoskeleton and scaffold-forming elements
Evidence has accumulated that contractile proteins contribute to the major nonhistone proteins (LeStourgeon, Forer, Yang, Bertram and Rusch, 1975; Douvas, Harrington and Bonner, 1975; De Martino, Capanna, Nicotra, and Natadi, 1980 and references therein; see, however also, Sanger, Sanger, Kreis and Jockusch, 1980). Yet no direct morphological evidence was obtained in the present studies that they are components of the scaffold. They should be extracted by the salt treatment, but could be left in a more unrecognizable aggregated form.
However, there are indications that another major cytoskeletal protein, tubulin, with its many "tubulin polymorphs," may be involved. Tubulin is usually associated with microtubulus-associated regulative proteins (MAPs), nucleotides, RNA and other substances (references in Dustin, 1978).
In SDS-PAGE gels of the scaffold, of both the chromosome bands and the NE (unpublished, see Plagens, 1978), one of the main gel bands is close to the molecular weight of tubulin subunits (about 55 000; see also Agutter and Richardson, 1980).
Tubulin can be associated with ruthenium red and lanthanum-staining material, probably polysaccharides, and is associated with carbohydrates (references in Dustin, 1978). Recent proof by immunological techniques and biochemical fractionation has localized tubulin in nuclei, chromosomes, and importantly, in the nuclear matrix (Menko and Tan, 1980). It has also been localized in the loops and axes of isolated lampbrush chromosomes by immunological staining (Karsenti, Gounon and Bornens, 1978). This nuclear tubulin must be in a non-microtubular form as has been claimed earlier (for references see Menko and Tan, 1980).
Tubulin can appear in many forms: as a pool of soluble subunits, as protofilaments, and certain ring forms, which can also be induced by different incubation conditions (Wallin, Larsson and Edström, 1977; Langford, 1978; Wiche, Honig and Cole, 1979; Voter and Erickson, 1979; and references in Dustin, 1978).
Further indirect evidence for nuclear tubulin includes colchicine-binding sites in chromatin and the NE (Stadler and Franke, 1972). Moreover, colchicine and other tubulin-binding drugs are known to modify the condensation and general appearance of chromosomes.
Using immuno reagents, heavy and light chains of myosin have been identified very recently in the NMPCL (nuclear matrix pore complex lamina), namely in the Mg/Ca-activated ATPase polypeptide fraction (Fisher, 1988). The myosin was suggested to be present as eight copies in both the nucleoplasmic and the cytoplasmic part of the NPC in a tail-to-tail orientation. This is based on the similarity in size and configuration to some of the NPC subunits. Good candidates would be the "claws" of the NPCs between the annular balls as described originally by Engelhardt and Pusa (1972a); for more details, see the model in Fig. 35. This suggestion, if valid, could not only explain wholesale nuclear migrations in developmental processes, e.g. in Drosophila as pointed out by Fisher (1988), but also the numereous observations on "nuclear rotation" in living cells with time-lapse studies. In meiotic cells, in addition to "nuclear rotation" intranuclear chromosomal movements can also be clearly demonstrated, during the chromosomal pairing process, especially at the zygotene-pachytene stages, using video time-lapse recordings (Engelhardt, unpublished). The cyclomere-associated myosin and available actin (regulated for instance by Ca2+ influx-effluxes) would in this way make intranuclear chromosome movements possible, especially cyclomere-directed movements (Engelhardt, 1978). The best evidence for the presence of contractile proteins in meiotic chromosomes, in addition to time-lapse studies, comes from indirect immunofluorescence demonstrations of myosin and actin in the synaptonemal complex (Capanna, De Martino, and Natali, 1980; De Martino, Capanna, Nicotra and Natali, 1980).
Many cytoskeletal proteins have a natural tendency to associate with regulative components. Phosphorylation and glycosylation of proteinsmay be involved in regulation. The NPC-lamina skeleton is reversibly depolymerized by phosphorylation (Gerace and Blobel,1980). There are several reports on endogeneous glycosyl-transferases in chromatin-depleted nuclei and the NE (see Agutter and Richardson, 1980, for references).
According to a recent report (Rottmann, Schröder, Gramzow, Renneisen, Kurelec, Dorn, Friese and Müller, 1987) a specific phosphorylation of NPC-lamina proteins occurs in the sponge Geodia cydonium produced by "the homologous aggregation factor" and phorbol ester; a role for protein kinase C was demonstrated in a 2.5-fold increase in the phosphorylation of DNA topoisomerase II. This is evidence for the presence of topoisomerase II also in the NPC-lamina fraction and not only in the scaffold (as discussed below).
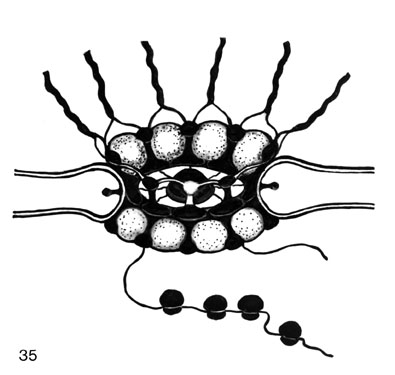
Fig. 35. A model of the NPC (nuclear pore complex). The apparatus is shown in perspective, whereas the nuclear envelope is represented in cross-section. The detachable nucleoplasmic portion (cyclomere) and front part of the cytoplasmic (receptor) portion of the NPC are in view. The upper set of annular balls (cyclomeres) and their chromatin-connecting fibres (CFs) is to be visualized on the reverse side. In front, the upper row of balls is depicted as transparent (and without CFs) to show the interior. The densified material, in black, consists of two zones, namely (1) the inner ring, (2) the outer NPC margin, and of (3) interpartition or claws which connect these zones, and finally (4) the central NPC granule or plug. The central granule connects with the inner ring by very thin fibrils. The space between two consecutive claws is tentatively envisioned as a sphere. The CFs connect somehow with these balls or rather with the claws and so do polyribosomes at the cytoplasmic side of the NPC. Lateral appendages protrude from the anticlinal membrane walls of the nuclear pore proper, into the perinuclear cistern. (Reprinted from Engelhardt and Pusa, 1974a; Engelhardt, 1976.
)
6. The elements of the band scaffold and topoisomerase II
Recently, the most successful way to characterize the proteins of the scaffold and the nuclear matrix has been by immunological techniques. These elegant methods showed that topoisomerase II is one of the proteins of the scaffold in metaphase chromosomes (Earnshaw and Heck, 1985; Earnshaw, Halligan, Cook, Heck, and Liu, 1985). This protein was also present in the nuclear matrix and intact polytene chromosomes of Drosophila (Berrios, Osheroff, and Fisher, 1985).
As mentioned in the introduction, some of the unexpected ultrastructural data on the scaffold elements fit with gyrase which is equivalent to topoisomerase II in prokaryotes; detailed structural data are not yet available on eukaryotic topoisomerase II. The core particles of gyrase have the same size in Pt-shadowed preparations (diameter 23 + 5 nm), and a spherical appearance (Moore, Klevan, Wang, and Griffith, 1983). However, according to the authors, these data did not fit with the theoretical calculation of the estimated volume corresponding to a diameter of 15 nm. It was concluded that the larger diameter, measured in microscopical preparations, probably resulted from flattening of the protein during drying and the deposition of metal.
These values fit surprisingly well with photogrammetric stereo measurements of the globular elements of the scaffold. These are also flattened being only half as high (10-15 nm) as wide (25-30 nm). It should be mentioned that these values were measured independently without knowledge of their fitness with those of gyrase.
Moreover, Moore, Klevan, Wang, and Griffith (1983) showed that gyrase has a natural, high tendency to complex with linear and circular DNA giving rise to rosettes with small loop size. These loops are clearly of the same size range that can be found with the band scaffold elements: monomers, linear and rosette (ring) form oligomers.
These data correspond to the loop-and-rosette model as presented below. They disagree completely with the 30-150 kb long loops in the "radial loop model" (reviewed by Paulson, 1988).
In addition there is an earlier report (Noguchi, veer Reddy and Pardee, 1983) on a multienzyme complex, called replitase, which included topoisomerase and DNA polymerase, besides nascent and template DNA and numerous enzymes required for DNA biosynthesis. In view of its possible relation to scaffold particles, it is interesting that the replitase fraction is also composed of spherical particles with a diameter of 24 to 30 nm in electron micrographs. The multienzyme activity was, however, noted to be restored only under milder conditions than those used in scaffold-matrix isolation.
7. DNA folding and scaffold elements
Hyaluronidase treatment not only helped to resolve the scaffold into its basic units but also made possible a much closer study of the intimate relationship of these elements to DNA folding than has been possible before (cf. Paulson and Laemmli, 1977; Mullinger and Johnson, 1979; McCready, Akrigg and Cook, 1979; Mullinger and Johnson, 1980). It is remarkable that in controls without hyaluronidase the histone-depleted loops (i.e. salt-treated) were long and it was not possible to see more details of how they were connected with the bulky main scaffold, whereas salt treatment followed by hyaluronidase gave, as one type of effect, a rosette-like folding with short single loops (Fig. 34). The first immediate explanation for this difference would be that incubation in the control buffer paradoxically results in a more complete unfolding of the rosettes, for some reason, e.g. by some endogenous enzyme activity. A further explanation is that the hyaluronidase treatment causes a loosening up of the scaffold and a release of the chromomeres. The rosettes would then be a reflection of a localized unfolding of chromomeric DNA (band DNA).
Altogether, hyaluronidase has some capacity to (1) unfold the DNA that is tightly packed, as in non-active band chromomeres, giving rise to rosettes with short loops. When the DNA stays packed, the effect is (2) bulky doughnuts and their linear transformations as thick short rods (the length of a doughnut periphery). When unfolding occurs, the hyaluronidase effect may also consist in (3) an arrangement of successive short loops on a core, which is obviously a straightened-out rosette.
Very similar rosettes, also with small loops, have been obtained in normal Chinese hamster ovary cell chromatin when the extraction was done at higher ionic strength, namely 4 M ammonium acetate (Okada and Comings, 1979, 1980). However, the core looked more aggregated and was not reported as ring-like after this mode of extraction.
As mentioned earlier, exceptionally long loops, 30-90 kb (10-30 mm), have been described in which the origin and end of the loop are close to each other on a compact scaffold (Paulson and Laemmli, 1977; review by Paulson, 1988). If all this effect is not just looping out of long interchromomeric DNA then either exceptionally long chromomeric loops have to be assumed or rather a total unfolding of a chromomere rosette into a single long loop by the action of the spreading forces (as pointed out earlier), or then by some endogenous enzyme which was kept in check by hyaluronidase and 4 M ammonium acetate in the other treatments. Such an endogeneous enzyme could be RNase (which is difficult to inhibit).
Indirect proof has been presented that structural RNA might be involved in the DNA folding (in eukaryotes and prokaryotes) together with proteins (Stonington and Pettijohn, 1971; Worcel and Burgi, 1972; Benyajati and Worcel, 1976). The folding of the DNA into rosettes and its attachment to the scaffold elements might be due to some RNA sequences, associated with proteins. These conclusions are not, however, supported by earlier results (Cook and Brazell, 1978).
In the absense of data from more thorough studies, alternative and quite contrary explanations must be considered. Specifically one could propose that the rosettes seen are artifactually formed by the action of hyaluronidase, and, in the other case, by the 4 M ammonium salt. There is actually indirect evidence in favour of this: under certain conditions, such as in the presence of polycations, DNA (of unknown purity? cf. Werner and Petzelt, 1981) has been reported to form compact doughnuts (toroids) (Widom and Baldwin, 1980). Some rosette-like foldings have been noted, not exceptionally, on mixing DNA and histones (Sonnenbichler, 1969 a, b; Comings and Okada, 1976) but they do not have a core with a definite organization, either ring-like or otherwise. To what extent hyaluronidase itself, a glycoprotein (Brunish and Högberg, 1960), or 4 M ammonium acetate can produce similar effects remains to be shown. Spreading DNA-protein complexes with cytochrome c can give rise to rod or doughnut-shaped particles and this is evidently produced by interactions with chromosomal proteins (tightly bound?) (Brack, 1981).
It has recently been claimed that, "rosettes" arise always and only when histone-depleted chromosomes (either interphase or metaphase) are pelleted and resuspended. "Rosettes" should therefore be considered artifactual (Paulson, 1988). This argument cannot apply to the mica method as no pelleting or resuspension steps are involved. In fact no gentler method of handling chromosomes is available at present. On the contrary, the spreading methods must be considered destructive in general. They can produce artifacts, that result solely from the spreading forces, as discussed above.
Although, rosettes and doughnuts could be produced artificially (cytochrome c replacing histones, for instance), as recently demonstrated in a detailed study (Leon and Macaya, 1983), they do not rule out that normal packing can, in fact, be something very similar. Artificial production could mimic the self-assembling and folding capacity inherent in the DNA sequences themselves.
In addition there are the "very tightly" (covalently?) bound proteins found in eukaryotic DNA (Werner and Petzelt, 1981; Neuer, Plagens, and Werner, 1983; Neuer and Werner, 1985; Chernokhvostov, Stel´mashuk and Razin, 1986; Avramova and Tsaneva, 1987). RNA fragments, as has been suggested earlier (Müller, Spiess, and Werner, 1983), or even polysaccharides could be linked to these proteins, as suggested by their unusual physical parameters such as buoyant density in CsCl (Chernokhvostov, Stel´mashuk and Razin, 1986). The particular nature of these proteins could be demonstrated in EM studies. It was recognized that their size and appearance were in close agreement with our earlier findings on 25-30 nm granular subunits of the matrix-scaffold (Engelhardt, Plagens, Zbarsky and Filatova, 1982).
The rosette-type folding reflects a most natural way of higher-order folding and packing of DNA for certain chromomeres. The strongest and most concrete argument is the fact that the core of certain rosettes is ring-like and can be seen separately, i.e. depleted of the DNA by digestion, as the independent ring-form alignment of scaffold units.
The recent data on the involvement of topoisomerase II in the scaffold (see previous chapter) give more variability to the DNA folding than could have been expected earlier. In fact, the contradictory effects of different incubation and treatment conditions on the loop length and complexity of the folding do have individual and specific explanations. It can be shown that gyrase (Moore, Klevan, Wang, and Griffith, 1983) when incubated with linear or circular (supercoiled or relaxed) DNA, can interact with many DNA sites on the same (or even separate) strands and that the stability of folding is very sensitive to the incubation conditions. Analogous data have recently been reported on the selectivity of supercoiled DNA binding sites in the nuclear scaffold (Tsutsui, Tsutsui, and Muller, 1988). Variable results on DNA folding and loop sizes within the scaffold may thus be superfluous until the experimental conditions are better define.
8. The loop-and-rosette model
The rosette arrangement agrees with our loop-and-rosette model which was based on thin-section studies (Engelhardt and Pusa, 1972, 1974a, b, 1975, 1977, 1978; Engelhardt, 1979). The model is presented in Fig. 36 for polytene chromosomes in revised detail (as compared with Engelhardt, Plagens, Zbarsky and Filatova, 1982) and for metaphase chromosomes in Fig. 37 (reprinted from Engelhardt and Pusa, 1974b, 1977, 1978, Engelhardt, 1979). This model may seem to have some resemblance to earlier chromosome models (reviews by Sorsa, 1969; Comings and Riggs, 1971; Dounce, Chanda and Townes, 1973; Paulson, 1988) in which hypothetical "DNA linkers" composed of nonhistones fold DNA pieces or the DNA strand into many loops (for recent proof on protein "tightly bound" with DNA, see: Neuer and Werner, 1985; Chernokhvostov, Stel´mashuk and Razin, 1986; Avramova and Tsaneva, 1987).
The "linkers" of the present model were earlier defined in concrete terms (Engelhardt and Pusa, 1972a; cf. Dounce et al., 1973). They can now be visualized as being organized from scaffold-forming elements. As the results clearly establish, unit elements of the scaffold can form different oligomers, linear or ring-form. Certain ring forms resemble in details the cyclomere, the nucleoplasmic part of the NPC. They presumably can attach to the NE at receptor sites, i.e. the cytoplasmic part of the NPC, by taking their definitive stable form as the NPC (Engelhardt and Pusa, 1972a, b). They can also detach, again as free "cyclomeres," as can be shown in sections (Engelhardt and Pusa, l972a, b, 1974a, b, 1975, 1977, 1978; Engelhardt, 1976, 1979; Pusa and Engelhardt, 1977).
If the DNA is continuous, a thread folded into many loops and rosettes is the most convenient way of folding the whole strand onto these underlying elements. In such a structure every loop can condense and decondense independently of the rest. Replication and segregation would not present problems: either all the length or any local stretch of the thread could, for topographical clearance, be simply transformed into a linear unfolded thread. According to the present findings, this kind of linear transformation occurs naturally, as indicated by the linear oligomer forms with loops protruding. In the model Figs. 36 A-C represent one chromatid as it is stretched out in the polytene chromosome and similarly Figs. 37 A, C in the metaphase chromosome.
According to our model for polytene chromosomes, lateral apposition of chromatids gives rise to the different degrees of band thickness (Fig. 36 D). Chromomeres stay laterally associated corresponding to size: single loops (extending from monomers), double loops and so on to octomeric loops, i.e. cyclomeres of the NPC and clusters of these. Other types of lateral interactions could cause constrictions, namely, underreplication (Laird, 1981), and the association of cyclomere-forming units from different chromatids under polytenization. Configurations suggesting the latter situation, i.e. several strands joined to a band chromomere, are found (V. Sorsa, pers. comm.). The other configuration observed is one strand joined with one chromomere. These situations are shown in the model (Fig. 35 D).
Originally in evolution, a single loop could have represented the primordial chromomere, which upon local duplication gave rise to the higher orders. It is easy to visualize that the loops after replication have been arranged in tandem and integrated into the chromosome (see Hourcade, Dressler and Wolfson, l973). These mechanisms do exist, as shown for the E. coli chromosome; what now is one replication unit, is the primordial unit duplicated twice during evolution (Zipkas and Riley, 1975). This must have happened in the many replicons of the eukaryotic chromosome as well.
These repeated duplications, i.e. loops in agreement with the "octagonal" NPC, may be a favoured situation in the evolution of eukaryotic chromosomes. The cluster of loops may be structurally related to the regulation of gene function. Besides the common initiator of replication and transcription (as suggested by Engelhardt and Pusa, l972a, b), the regulation in the loop system would involve duplications, i.e. variations in the number of replicons.
If a loop is fully stretched, the average length has been estimated to be no more than a few (l.2-2.2) kb (kilobase). Contraction of the small loops when associated with nucleosomes (6-11 per loop) can simply make superbeads (Hozier, Renz and Nehls, 1977; Jorcano, Meyer, Day and Renz, 1980) or supranucleosomes (Scheer, Sommerville and Muller, 1980). The loop length is one or even two orders smaller than observed for the replicons of eukaryotes (Paulson and Laemmli, l977, and references therein). The rosette as a whole, therefore, probably corresponds to the replicon. The replicon length varies inversely with the rate of proliferation (references in Harland, 1981), and polyteny would presumably represent rapid proliferation of chromatids.
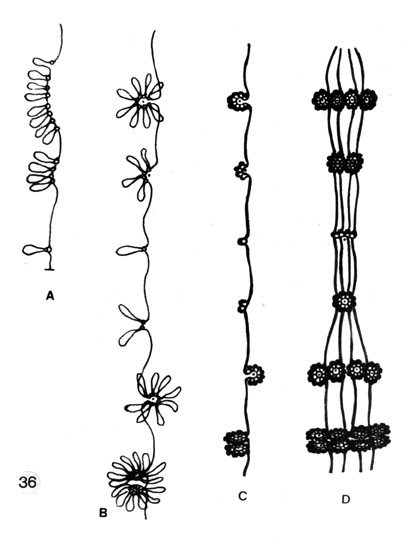
Fig. 36. Loop-and-rosette model of higher-order DNA folding in polytene chromosomes; chromomere cores, single or compound (interpreted as scaffold elements).
A. Single elements (cyclosomes-globosomes) plus single histone-depleted DNA loops and accumulations (duplications) of these in linear form.
B. Arrangement of the grouped scaffold element-loops into compact ring-like chromomeres (cyclomeres with loops). The packages, from single element-loops to cyclomeres, represent various size classes and organization types of chromomeres.
C. As B but a native state: the small DNA loops (l.2-2.2 kb), when associated with 6-11 nucleosomes, are condensed further around the scaffold elements giving rise to superbeads (Hozier, Renz and Nehls, 1977; Jorcano, Meyer, Day and Renz, 1980) or supranucleosomes (Scheer, Sommerville and Muller, 1980).
D. Polytenisation: lateral apposition of chromatids (only 4 chromatids are drawn for clarity to show the principle), demonstrating the formation of bands of different thickness. A constriction is interpreted as reunion of globosomes from separate chromatids so as to form cyclomeres, or as a tendency for small chromomeres to fuse, rather than as a point of underreplication (cf. Laird, 1981).
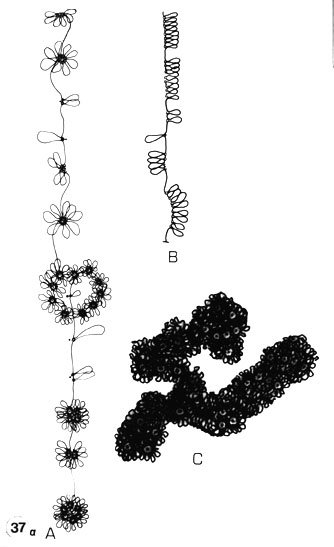
Fig. 37 a. The principles of the loop-and-rosette model in metaphase chromosomes.
A. The folding of a continuous DNA by looping: single loops or monomers which would correspond to simple NE attachments; double loops (local duplication), multiple loops up to octamers (rosettes), i.e. cyclomeres, and their accumulation; representing the origins of different-sized chromomeres during evolution. The strand represents a partly stretched out (interphase) chromatid.
B.Total or strictly local unfolding of chromomeres in a chromatid into a linear form. Replication is easily envisioned in this form.
C. The condensed form which represents the metaphase condensation. The cyclomeres are embedded in the chromatin, the loops more condensed, thicker (cf. the similarity in appearance of whole-mounted CHO metaphase chromosomes in Results II Fig. 5 and a section of the premeiotic X chromosome of Acheta in Fig. 37 b).
There is a complete analogy with a chromatid of the polytene chromosome. Only the condensation is different as no lateral apposition of chromatids occurs. Instead, a linear apposition causes chromatid coiling (thick arrow) and linear condensation of the chromosome arms. This is presumably not only due to interchromomeric DNA condensation into a 30-nm thick chromatin thread but rather to the tendency of the core elements (scaffold elements, cyclomeres and their subunits) to interact and assemble. This results in higher-order structures like chromatid coils, in which these ring-like assemblies are arranged like rings on a string (as can be visualized in critical point-dried whole-mounts in stereo inspection, Results II: Fig. 5). (Reprinted from Engelhardt and Pusa, 1974b, 1977, 1978; Engelhardt, 1979.)
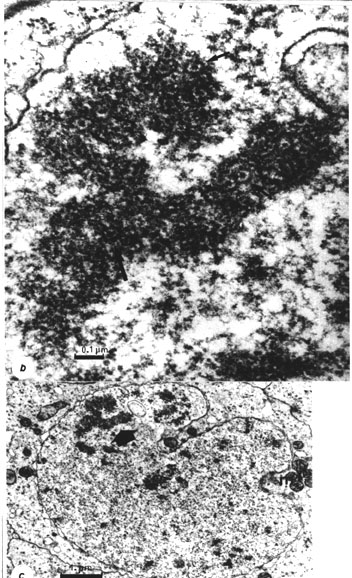
Fig. 37 b. A section through the premeiotic X chromosome of Acheta (fixed with the "successive ruthenium red" method in Ringer solution, Engelhardt, 1976, 1979). Note the separate chromatids which resemble a metaphase chromosome with cyclomeres visible (arrows).
Fig. 37 c. A general view of the same nucleus to show the position of the X chromosome in the nucleus. (Figs. 37 b, c reprinted from Engelhardt and Pusa, 1974b; Engelhardt, 1979)
Academic Dissertation 1988
Eukaryotic chromosome structure
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
Peter Engelhardt
Email: Peter.Engelhardt@Helsinki.Fi
Available at http://www.csc.fi/jpr/emt/engelhar/Doc/Diss-DiscI.html



