II. ACID-ISOLATED METAPHASE CHROMOSOMES, INTERPHASE NUCLEI AND POLYTENE CHROMOSOMES
The development of new techniques for analysing metaphase chromosomal sets in EM for cytogenetical purposes (Engelhardt, in prep.), resulted in excellently preserved whole mounts of metaphase chromosomes, interphase nuclei and polytene chromosomes. A "scaffolding" which holds the shape of the chromosomes, was preserved, although these samples were obtained by harsh treatments. These preparations served to check the results obtained with manually isolated polytene chromosomes and nuclear envelopes.
1. Isolation of prefixed metaphase chromosomes and nuclei with acid treatment
These techniques originated from spreading techniques developed for unfixed polytene chromosomes (Burkholder, 1976; Kalisch and Hägele, 1981). When material, conventionally prepared for cytogenetical purposes (hypotonic treatment and methanol/acetic acid fixed), was further washed with a (66%) PA/CA mixture (Kalisch and Hägele, 1981) or more simply by 60% acetic acid, metaphase chromosomes and nuclei were completely released from all adhering cytoplasmic fragments (cf. Ris, 1981).
Inspection by phase-contrast light microscopy showed very clean and morphologically excellently preserved metaphase chromosomes and interphase nuclei. As a criterion, for instance in CHO metaphase chromosomes, chromosome (chromatid) coiling could be easily detected at the light microscopic level.
Upon EM inspection of either spread, centrifuged or mica-mounted whole mounts of metaphase chromosomes, some slimy, obscure material on the chromosomes was still visible making them diffuse and unsharp in ultrastructural appearance. This material could be further abolished by washing the chromosomes (attached to plastic-coated grids or mica) with 50% acetic acid at 100 oC, for 30-60 s (modified from Welter, Black and Hodge, 1984, for scanning electron microscopy of chromosomes). This treatment had no other detectable influence on the gross morphological appearance of the chromosomes or nuclei.
2. Scaffold particles in the isolated chromosomes
The finding of scaffold particles was another criterion that showed that even the fine structure of the chromosome scaffold was well preserved after strong acid treatment.
Metaphase chromosomes spread on distilled water and post-treated with hot acetic acid showed, unexpectedly, globular particles which protruded out on a zone around the chromosome axis in air-dried spreadings before (Fig. 1), or more clearly after, rotary Pt shadowing (Fig. 2). These particles were very similar in size and appearance to those earlier described (see above Results I).
The particles could also be shown on critical point-dried whole mounts on mica, especially after DNase treatment (compare Figs. 3 and 4). The residual chromosomes were clustered with similar particles including the remnants of the double minutes (Fig 4). Before DNase treatment both the chromosomes and the double minutes showed very clear clusters of chromatin loops that disappeared after digestion. Note also the difference in the distribution of material, as there was no zone around the critical point-dried whole-mount chromosomes as compared with spread chromosomes.
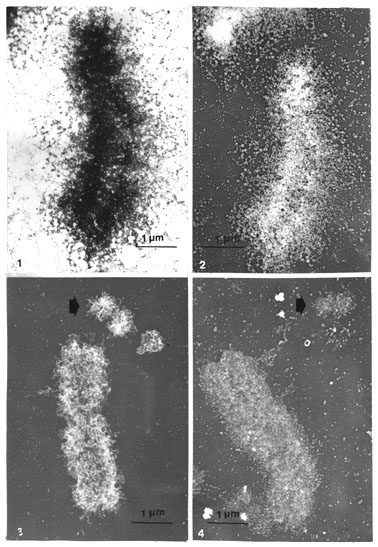
Figs. 1-2. Acid (PA/CA) isolated prefixed metaphase chromosomes (elk) spread on water, postwashed on plastic-coated grid with hot 50% acetic acid, dehydrated, stained with uranyl acetate in methanol and air dried. Globular (scaffold) particles can be distinguished effusing in a zone around the chromosomes axes, especially after rotary Pt shadowing of the same chromosome (Fig. 2) (x 14 000).
Figs. 3-4. Pt-carbon replicas of critical point-dried whole mounts of metaphase chromosomes (human COLO 320, containing double minutes) on mica (EMC method). Clusters of chromatin loops are very prominent in metaphase chromosomes and in the double minute chromosome (thick arrow) in Pt shadowing before DNase digestion. These loops disappear after DNase and globular (scaffolding) particles are uncovered (x 14 000).
3. EM stereo inspection of whole-mounted, critical point-dried metaphase chromosomes and nuclei
Intimations of ring-like assemblies of the particles could, with some difficulties, be detected in the air-dried spreadings and in the critical point-dried Pt-shadowed whole mounts.
Internal ring-like structures could, however, be best shown in stereo pairs of the original negatives (or positive transparencies) of whole mounts of critical point-dried chromosomes, washed in hot acetic acid as above, with only uranyl acetate staining3 (omitting Pt shadowing as a covering Pt layer is too dense and obscures internal structures).
In this series of pictures (Figs. 5 a-e), presented as three sets of stereo pairs, the chromatid coiling, already detected with phase contrast in LM, can be seen. Furthermore, the gyres or coils can be interpreted as being composed of ring-like structures aligned like rings on a chain. They appeared on the coils either as empty holes, specially in the more dense regions or in lighter regions as rings, in which sometimes even a central granule could be vaguely envisaged. Most importantly, in some favourable situations, at the right tilting angle and location as in some peripheral parts, the granular subunits of the rings could clearly be shown (Fig. 5 d, e and Fig. 5 f, details at higher magnification). In Fig. 5 f, even short loops can be detected, where both the loop origins can be seen protruding from the same particle (cf. Figs. 34 b, c, d).
The gradual change of tilting angle, with steps of 6 degrees, all through + 60 degrees [Fig. 5 g, h (0 to + 60 degrees)], showed the three-dimensional appearance of the chromatid coiling at different angles (viewed in stereo). The appearance and disappearance of the ring-like structures were also evident when the tilting angle was changed, demonstrating the delicacy of the structures and the importance of the degree of the tilting angle in relation to the magnification and specimen thickness (Turner, 1981 and further references therein).4
As mentioned above, similar-sized pore or ring-like structures are more difficult to see in Pt-shadowed critical point-dried whole mounts. This results from the Pt covering which enhances everything equally and also from the fact that the protruding clusters of chromatin loops effectively hide the internal rings. Without Pt the chromosomes are more transparent causing the chromatin loops and scaffold structures to be distinguished.
In any case the ring-like structures can be detected with Pt shadowing in stereo pairs in places of chromosomes where they are occasionally more exposed (Figs. 6 and 7). For example, in Fig. 7, where one arm of a smaller human chromosome is partially coiled, a row of two or three rings can vaguely be seen. Rings and their clusters can be seen but as they were not more demarcated or distinctly stained it was difficult to distinguish them from the protruding loops of chromatin.
These chromosomal "scaffolding" ringlets may be directly compared with those of the NE, in critical point-dried whole mounted nuclei in the same preparations and stereo pairs. Figs. 8 a, b, and c present one nucleus and two chromosomes.5 In the nucleus residual NPCs can be seen. Presumably only the nucleoplasmic part of the NPC was preserved, as it is difficult to distinguish complete (bipartite) NPCs even at the bottom where the NE is flattened (appearing partly in upper left and lower right corner of the nucleus). The NE looks like a network of residual pores which can be distinguished as small but equal-sized rings which can be seen inside both chromosomes. This inspection is very clear-cut with both stereo viewing methods (particularly so with the original negatives), simply proving this relationship in the same picture at one glance.
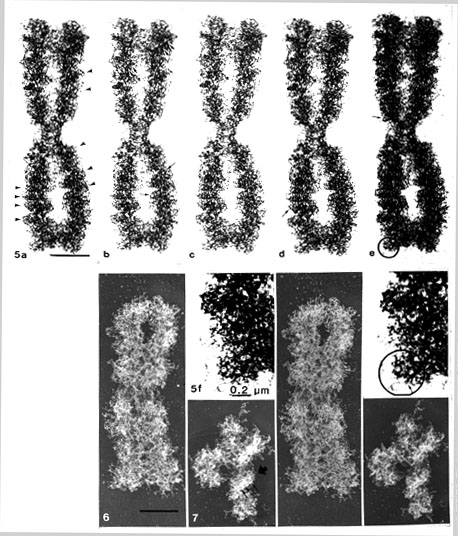
Figs 5. (a-f). Critical point-dried whole mounts of CHO metaphase chromosomes (WMC method, no Pt shadowing): a, b,
-12o; c, d, -6o; e, 0o tilting angle. The prints are slightly differently exposed for comparison. Mounted as three sets of stereo pairs (a and c; b and d; c and e). Chromosomal (chromatid) coiling is distinguishable (arrowheads). Ring-like structures with 60-80 nm inner, and 90-110 nm outer diameter oriented like rings on a chain (small arrows), can be found in these coils, in stereo inspection. In the denser areas, they are seen as pores or empty holes and in lighter regions as rings. In some places (encircled) rings with granular subunits can be detected, clearly seen at higher magnification (Fig. 5 f).
3 There were some difficulties, however, to make prints where all details could be clearly seen as they tend to become too black even with the softest paper available. This discrepancy could be partly solved by making a series of prints with slightly differing gray-scale shadings.
Fig. 5 f. Stereo pair. Note that chromatin loops can be detected protruding from some particles (small thin arrow)
(a-e, x 13 800); (details 5 f, x 33 200).
Figs. 6 and 7. Human metaphase chromosomes (human COLO 320) prepared as in Fig. 3, on mica (EMC method). Stereo inspection of critical point-dried Pt-shadowed chromosomes, showing the difficulty in distinguishing scaffold ringlets from their clusters of protruding chromatin loops. However, in favourable situations (Fig. 7) where distinct chromatid macrocoilings (thick arrowhead) are seen, a row of scaffolding pore or ring-like structures are vaguely exposed (thin arrows) (x 13 800).
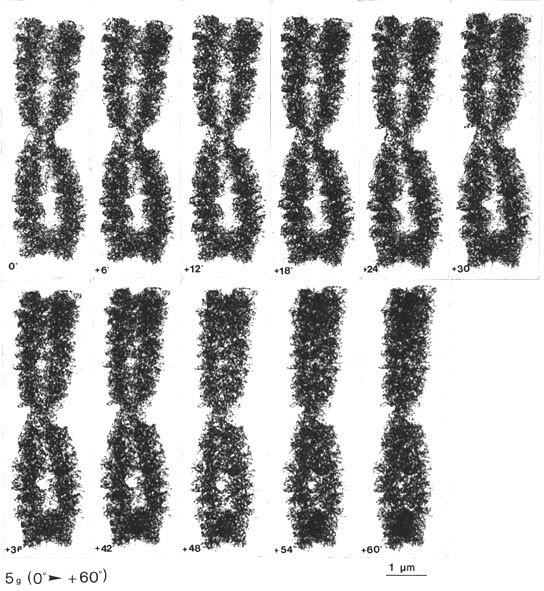
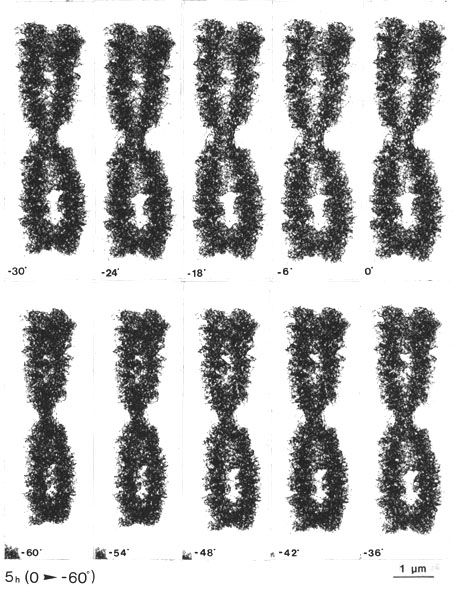
Fig. 5 g, h. The same CHO metaphase chromosome tilted through 0 + 60o, with steps of 6o. The gradual appearance of the chromatid macrocoiling and the pore and ring-like structures can be followed from different angles (every second picture can be viewed as a stereo pair, with a total tilting angle of 12o; see, however, the comment in footnote 4 (x 13 800).
4 Note that in Figs. 5 g, h every stereo pair (i.e. every second picture) has a total tilting angle of 12 degrees, which (at this magnification) seems slightly too large for proper visualization of the details. Note that it is only 6 degrees in the stereo pairs of Figs. 5 a-e (with the same magnification, for comparison).
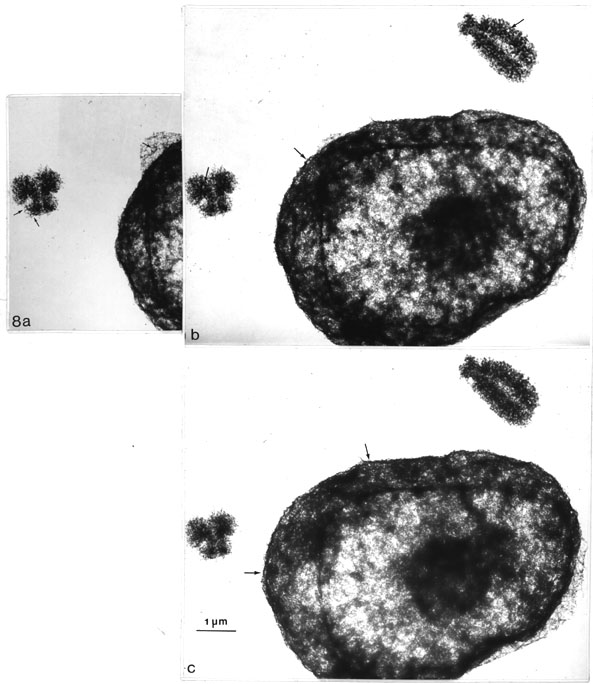
Fig. 8 a-c. Direct comparison of critical point dried CHO metaphase chromosomes and nuclei (WMC method, no Pt shadowing). The pictures can be viewed in stereo in two different ways (see the comments in footnote 5). The scaffold ringlets seen inside both chromosomes resemble in size and appearance those that can be detected on the surface of the nuclei (thin arrows) representing residual NPCs. Figs. 8 a, b can be viewed with an ordinary pocket stereoscope (2-3 x magn.). Figs. 8 b, c as explained in footnote 5 (x 13 800).
5 These pictures can be viewed by two different stereoscopic methods: Either like earlier strereo pictures side by side, horizontally (Figs 8 a, b) with a pocket stereoscope (magnification about 3x) or with a new device for which the pictures are aligned vertically (Figs 8 b, c) and viewed with special prism-glasses (System Nesh: Stereo-Vertrieb Nesh, Münster, W-Germany). The advantage of this system is that larger pictures can be viewed. The disvantage is that no additional magnification is available and the pictures must be viewed from about one armlength´s distance, which makes the details even smaller (for references see Turner, 1981).
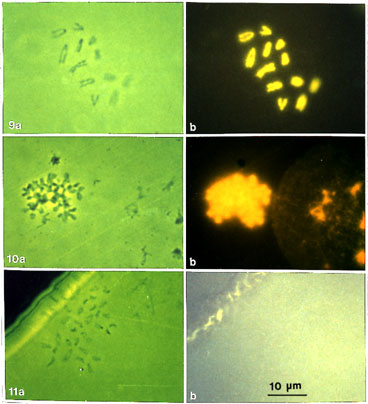
Figs. 9-11. General description. Phase-contrast and fluorescence pictures of spread metaphase chromosomes (elk) and nuclei on grids, stained with DAPI and embedded in DAPI-containing mounting medium, before and after DNase digestion, and EM processing. All pictures are of the same magnification (x 1 300).
Figs. 9 a, b. Control preparation of prefixed metaphase chromosomes after pretreatment (PA/CA) and spreading on U/HCl hypophase. The grid was stained and embedded as described above before EM processing. (a) phase contrast and (b) fluorescence picture of the same chromosome set. Chromosomes give an intense fluorescence with the DNA-specific fluorescence dye DAPI demonstrating that the DNA is preserved.
Fig. 10 a, b. A further control preparation. Prepared as above but spread on water. The grid has been further washed sequentially in hot 50% acetic acid, 2 M salt solution, TM buffer, 2 M salt solution, stained and embedded as above. In this picture spread interphase nuclei are included with a metaphase chromosome. (a) phase contrast and (b) fluorescence picture, slightly over-exposed to show the fluorescence of a thinly spread interphase nuclei clearly. An intense fluorescence proves that DNA is still preserved after these treatments.
Fig. 11 a, b. DNase-digested chromosomes. Prepared as in Fig 10, but including the DNase digestion (1h) step after the first 2 M salt washing. Further washing, staining and embedding as above. (a) in phase contrast a typical chromosome set can be seen but (b) in fluorescence no detectable DNA can be seen. The picture was purposely over-exposed to enable detection of the slightest fluorescence.
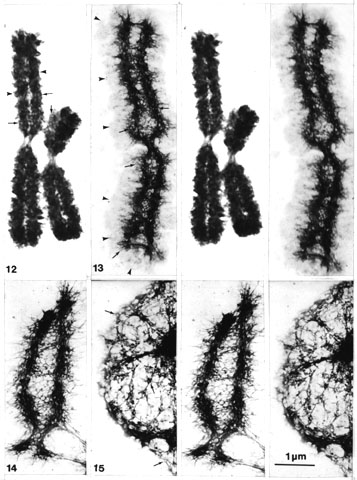
Figs. 12-15. General description. EM stereo pairs of spread CHO metaphase chromosomes before and after DNase digestion and compared to similarly digested interphase nuclei. All pictures have the same magnification (x 13 800).
Fig. 12. A control preparation of acid (PA/CA) isolated prefixed CHO metaphase chromosome spread on a cytochrome c solution. After spreading the grid was washed in 2 M salt solution, dehydrated, stained in uranyl acetate in methanol and critical point-dried. The chromosomes stay very condensed and chromatid coiling can be seen (arrowheads). However, in the condensed coils lighter pore-like areas (small arrows) can be detected (especially in stereo), similar to the ring-like structures described earlier.
Fig. 13. A further control preparation, as above (Fig. 10), but after spreading and picking up the preparation, the grid was washed with hot 50% acetic acid and then processed as described. A halo of diffuse chromatin (arrowheads) surrounds a chromosomal scaffold structure (cf. Paulson and Laemmli, 1977). The chromosome scaffold is composed of a network of pore or ring-like structures (small arrows).
Fig. 14 and 15. Spread as for the control preparations above but digested with DNase. The scaffolding structure of the chromosome stays intact with a similar network of pore or ring-like structures (small arrows), but the halo of diffuse chromatin disappears and shows no detectable DAPI fluorescence in light microscope (cf. Fig. 11). The pore or ring-like structures (small arrows) are similar in size and appearance to those found in the NE (and nuclear matrix) of similarly treated nuclei in the same preparations (Fig. 15).
4. Salt and DNase treatment of spread metaphase chromosomes and nuclei
These descriptions could be taken to refer to irrelevant chromatin configurations, if it were not for the fact that these pore and ring-like structural networks were resistant to 2 M salt extraction and digestion with nucleases and shown to be completely devoid of DNA (as checked by staining with DAPI).
Different preparations on grids, with chromosomes and nuclei, using any of the spreading or whole-mounting methods, and washing steps (see Material and methods), or control preparations extracted with 2 M salt solutions and buffer solutions used in DNase digestions (without DNase), gave an intense fluorescence when stained with DAPI (Figs. 9 a, b, 10 a, b), on the same grids taken later to EM inspection.
By contrast, only a short digestion with DNase (or DNase and RNase), before and after 2 M salt extraction, abolished all fluorescence. In phase-contrast microscopy, however, residual chromosomes can clearly be seen (Figs. 11 a, b) on the grid.
On EM inspection of metaphase chromosomes and interphase nuclei, after any of the various spreading and washing methods, and after 2 M salt extraction before or after DNase digestions, networks of ring-like structures were uncovered, in the residual (scaffolding) parts of the chromosomes.
The chromosomes became very condensed in control preparations, spread on cytochrome c solution and washed with 2 M salt. Chromatid coiling could be detected and more importantly, when inspected in stereo (Fig. 12), the coils showed lighter pore-like areas of the same size as the ring-like structures described above.
In further control preparations washed with hot 50% acetic acid solution followed by 2 M salt treatment, a typical scaffolding structure (cf. Paulson and Laemmli, 1977) is uncovered, with networks of pore or ring-like structures (Fig. 13) surrounded by a diffuse area of chromatin fibres.
This diffuse halo of chromatin disappeared during DNase digestion but the scaffolding structure remained intact (Fig. 14) with a similar perforated network of pore or ring-like structures. These appeared identical to residual pores found on the NE, and the internal matrix of similarly treated interphase nuclei (Fig. 15), which were found on the same grids.
5. Spread polytene chromosomes
What has previously been said about metaphase chromosomes also applies to the polytene chromosomes. However, suspensions of free polytene chromosomes have not been achieved using these techniques. Therefore the only way to release the chromosomes from the nuclei was by using spreading methods.
Looking at polytene chromosomes, spread according to Kalisch and Hägele (1981), indicated that all bands were composed of tight clusters of similar pore or ring-like structures shown here in Fig. 16 in stereo inspection. The enormous bulkiness of the bands in these chromosomes caused some difficulties in visualizing them in prints. This problem could be partly solved by limiting the inspection to very thin bands, peripheral parts of thicker bands or telomeric regions. In any case, from detailed stereoscopic studies, one can draw the conclusion that the bands are composed of structures similar to the pore-perforated networks found in the scaffolds of spread metaphase chromosomes.
With some modification of the method above, especially inclusion of washing in hot 50% acetic acid (Material and methods: II. 4. steps 2 and d; dehydrated, stained and critical point-dried), a well preserved three-dimensional structure of polytene chromosomes was obtained which is analogous with, although bulkier in size, than the critical point-dried whole mounts of metaphase chromosomes (Figs. 17 a-d; viewed in stereo, see footnote 5). However, ring-like structures were not as easily detected in the band regions of these bulky preparations; in the peripheral parts of bands (Fig. 17 c; and d, details at higher magnification) they were visible. In some of these rings granular subunits could be seen which were identical to those already detected in the critical point-dried whole-mounted metaphase chromosomes (cf. Figs. 5 f, and 17 d). Although it is somewhat difficult to visualize in prints, this indicates that the rest of the densely stained bands are tightly packed clusters of ring-like structures which are of the same type that was found in the metaphase chromosomes.
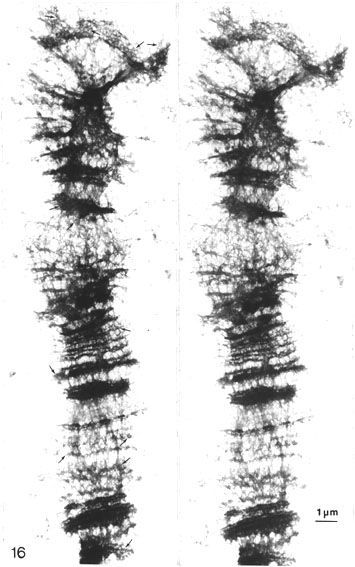
Fig. 16. Stereo pair of a polytene chromosome (Drosophila) spread according to Kalisch and Hägele (1981) and EM processed. Inspection in stereo indicates that all bands are composed of tight clusters of similar pore or ring-like networks (thin arrows) as in the scaffold of metaphase chromosomes. This can be revealed by studying telomeric regions, thin bands or peripheral parts of thicker bands of these bulky chromosomes (x 7 800).
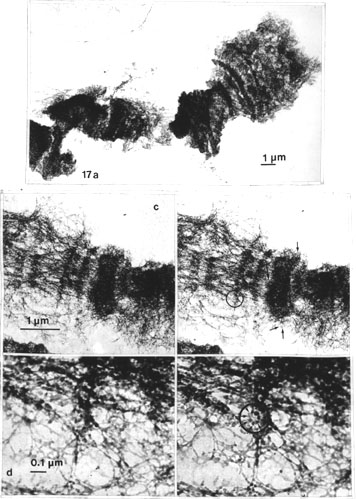
Figs. 17 a-d. Similarly pretreated (PA/CA) polytene chromosome but with some minor changes. This includes: a wash in 60% acetic acid before spreading on water. The grid is further washed in hot 50% acetic acid, dehydrated, stained and critical point-dried. This whole mount of a polytene chromosome is analogous with the metaphase chromosomes obtained by the WMC method (cf. Figs. 5).
Fig. 17 a. General view of a segment of a critical point-dried polytene chromosome.
Fig. 17 c, d. Stereo pairs of details at higher magnification. In the periphery of Fig. 17 c (viewed with pocket stereoscope 2-3x magn.) ring-like structures (thin arrows) can be seen and in some rings (encircled) granular subunits can be detected, especially at higher magnification (encircled and thin arrows, Fig. 17 d). Ring-like alignments of scaffold-forming particles can be visualized in the bands, and they are similar to those found in metaphase chromosomes (cf. Fig 5). This indicates that polytene chromosome bands are composed of the same scaffolding ring-like structures and subunits as metaphase chromosomes and this is best shown when the gross structure of the bands is also well preserved. (Figs. 17 a, x 4 500; c, x 13 800; d, x 52 000.)
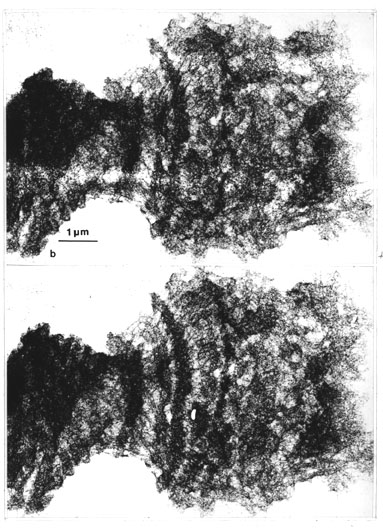
Fig. 17 b . Stereo view (see the comments in footnote 5) to show the bulkiness of these polytene chromosomes therefore the pore-like structures are only vaguely visualized (x 13 800) .
6. Salt and nuclease treatment of spread polytene chromosomes
As with the case of metaphase chromosomes, these descriptions could refer to merely peculiar morphological coincidences in chromatin, if these structures were not also completely resistant to 2 M salt extraction and DNase and RNase digestion.
In control preparations of spread polytene chromosomes on grids (washed in 2 M salt solution) a very distinct fluorescence with DAPI could be seen (Figs. 18 a, b). This staining was completely abolished after further DNase and RNase digestion. However, these chromosomes could clearly be seen in phase contrast (Fig. 20 a, on a grid mounted in the DAPI-containing medium) but not with fluorescence (Fig. 20 b).
Inspection of control grids in EM (before 2 M salt and DNase treatment) showed pore or ring-like structures, again detectable more abundantly at the periphery of the bulky band remnants (Figs. 19 a, b).
It was most remarkable, however, that after 2 M salt, DNase and RNase treatments plus further washing with 2 M salt, the gross structure of the polytene chromosomes stayed apparently the same (although no detectable DNA was present). In these preparations the sponge-like network, with rough fibres or cables, was very typical, and probably enhanced further by the spreading forces (and the pretreatment at low pH?). Pore or ring-like structures could be detected (Figs. 20 c, d) and compared to the residual NPCs of some NE fragments, adhered to certain regions of the polytene chromosomes.
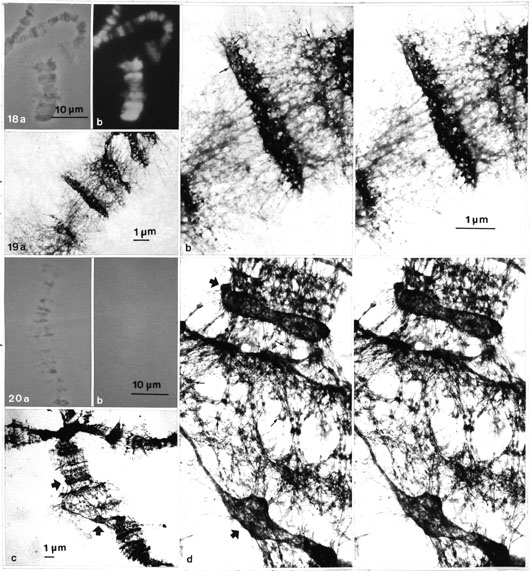
Figs. 18 a, b. Light micrographs: (a) phase-contrast and (b) fluorescence pictures of spread (according to Burkholder, 1976) polytene chromosomes on grid. The grid has been further washed in 2 M salt solution and embedded in DAPI-containing mounting medium, before EM processing. The chromosomes give a distinct DNA fluorescence with DAPI showing that DNA is fully preserved in control preparations before DNase digestion (cf. Figs. 20 a, b) (x 1300).
Figs. 19 a, b. General view (a) and a stereo pair (b) of a methanol/acetic acid prefixed, 60% acetic acid post-treated and water-spread polytene chromosome after the grid has been dehydrated, stained and air dried. The general view is somewhat diffuse (without the hot 50% acetic acid wash); nevertheless, in the densely stained bands pore or ring-like structures (thin arrows) are detectable in stereo inspection, particularly in the periphery (a, x 5 100; b, x 13 800).
Figs. 20 a, b. Light micrographs of a spread polytene chromosome treated as the control preparation above (cf. Figs 18 a, b) but including nuclease (DNase and RNase) digestion (1 h). The chromosome can be seen in phase contrast (a) but not in the fluorescence picture (b), on the grid embedded in the DAPI-containing medium before EM processing (x 1 300).
Figs. 20 c, d. Electron micrographs of a digested (DNase and RNase) polytene chromosome: (c) general view, (d) stereo pair. Note that the gross structure of the whole chromosome is apparently intact, although no detectable DNA (DAPI fluorescence) is present. Details are, however, more diffuse presumably because treatment with hot 50% acetic acid was not included. Still, pore or ring-like structures (thin arrows) can be seen and compared to those found on the residual NE fragments (thick arrows) stuck to some chromosomal regions as revealed by stereo inspection (c, x 3 200; d, x 13 800).
Academic Dissertation 1988
Eukaryotic chromosome structure
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
Peter Engelhardt
Email: Peter.Engelhardt@Helsinki.Fi
Available at http://www.csc.fi/jpr/emt/engelhar/Doc/Diss-ResII.html










