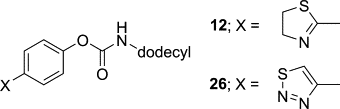|
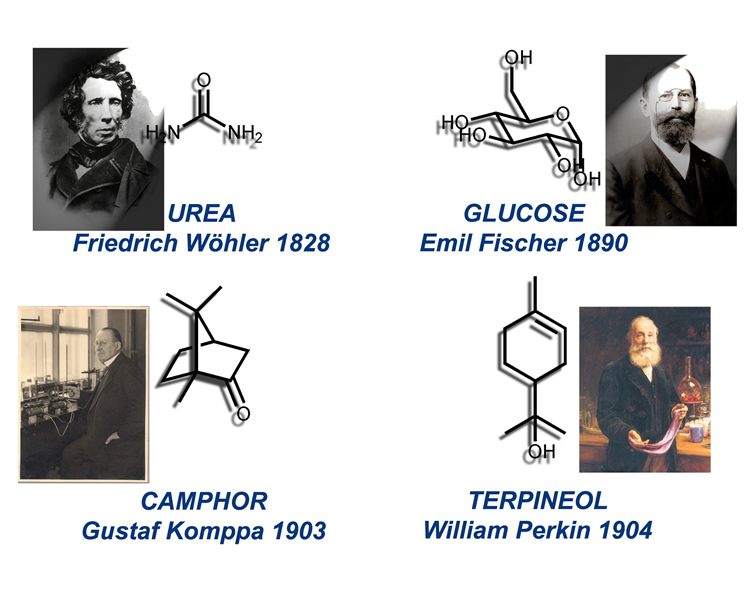
Welcome to visit the pages of the Koskinen Group. The Polytechnic School of Finland obtained the University status as the first Technically oriented University if the country in 1908, and 14 professorships were initially founded. Among them was one chair in chemistry, that of Organic Chemistry, which was held by Gustaf Komppa, who had a few years earlier managed to synthesize camphor in a landmark synthesis, which later has been considered as one of the pioneering syntheses of complex natural products, comparable to the syntheses of urea, glucose and terpineol.
Natural product synthesis has been the major theme of the Laboratory of Organic Chemistry in our institute ever since, as is obvious from the list of holders of the prestigious Komppa Chair. 1848-1874 Anders Olivier Saelan Recent publications: ________________________________________________________________________________
Pelss, A.; Koskinen, A.M.P. ’Stereocontrolled Construction of Decahydroquiinoline Ring Systems: The Case of Lepadin Alkaloids.’ Khim. Heterocykl. Soedin. 2013, 249–263. ________________________________________________________________________________
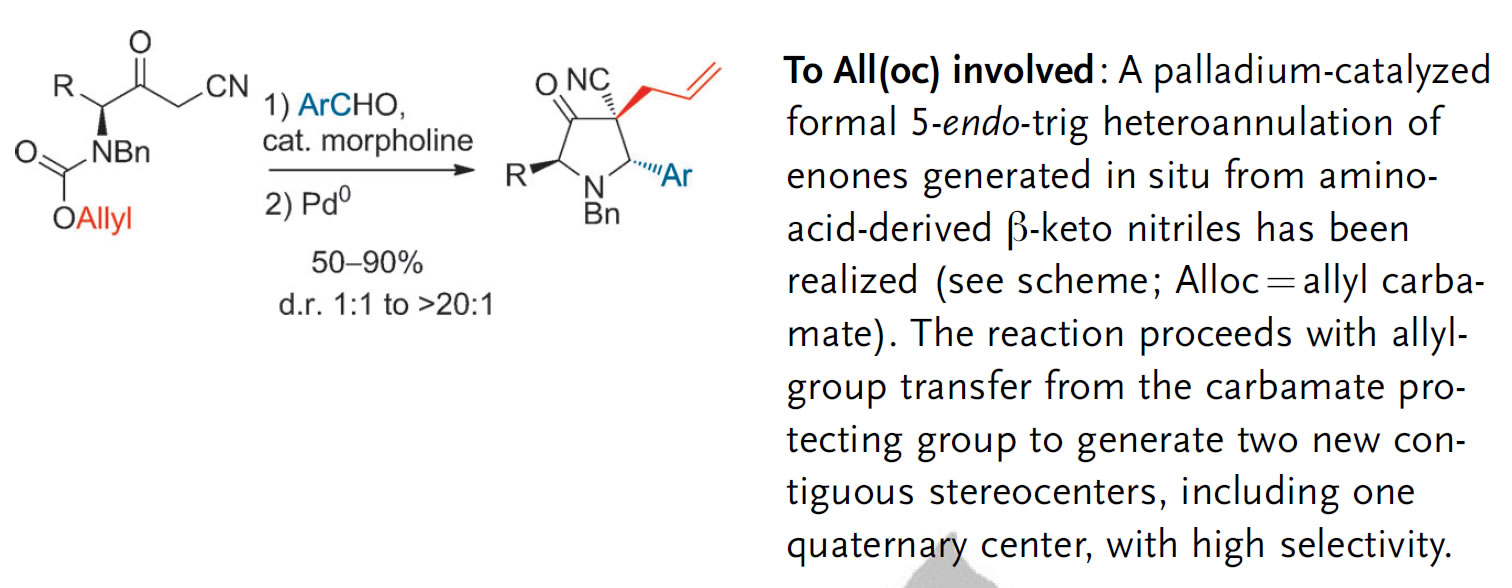
Karjalainen, O.K.; Nieger, M.; Koskinen, A.M.P. ‘Diastereoselective intramolecular allyl transfer from allyl carbamate accompanied by 5-endo ring closure.’ Angew. Chem. Int. Ed. 2013, 52, 2551–2554. ________________________________________________________________________________
http://eu.wiley.com/WileyCDA/WileyTitle/productCd-1119976685.html Natural product synthesis has played a key role in the development of many synthetic methods and will continue to do so in the future. Many recent advances in such diverse fields as immunology, cellular biology and materials science have been achieved through the synthetic chemist's ability to construct often very complicated structures in one enantiomeric form. Asymmetric Synthesis of Natural Products, 2nd Edition introduces students to this rapidly growing field of organic chemistry. The initial chapters present the foundations of asymmetric synthesis, including the theory and applications of individual asymmetric reactions. This is followed by chapters on each of the major individual classes of natural products; their structures, biosynthesis and interrelationships as well as examples of asymmetric syntheses and the practical value of these compounds. Natural product classes covered include carbohydrates, amino acids, peptides, proteins, nucleosides, nucleotides, nucleic acids, polyketides, isoprenoids, shikimic acid derivatives and alkaloids. For this second edition the text has been thoroughly updated and expanded, and includes new discussions and examples covering atom and redox economies, practical aspects and environmental awareness. Organocatalysis has emerged completely in the last ten years, and has been fully integrated into this new edition. Asymmetric Synthesis of Natural Products, 2nd Edition will find a place on the bookshelves of advanced undergraduates and postgraduates working in natural products chemistry, organic synthesis, medicinal chemistry and drug discovery. It is also useful for practising researchers who want to refresh their knowledge of the field. ________________________________________________________________________________
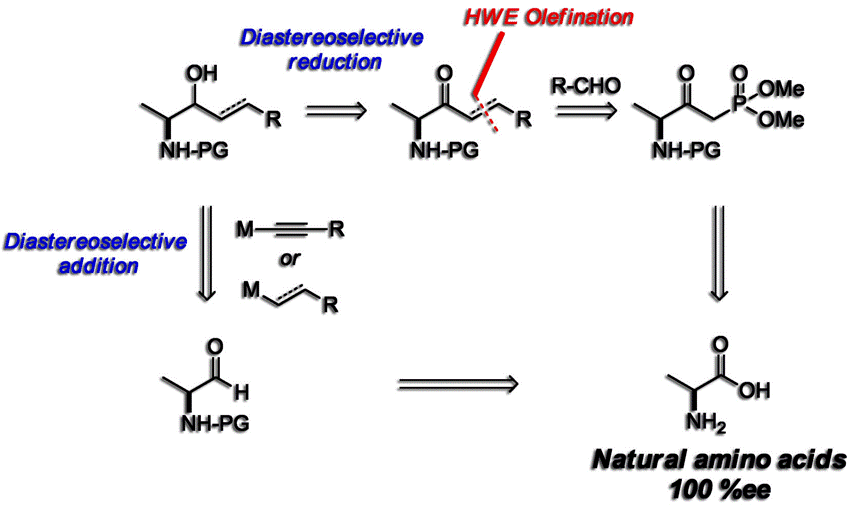
Karjalainen, O.K.; Koskinen, A.M.P. ‘Diastereoselective Synthesis of Vicinal Amino Alcohols.’ Org. Biomol. Chem. 2012, 10, 4311–4326. ________________________________________________________________________________
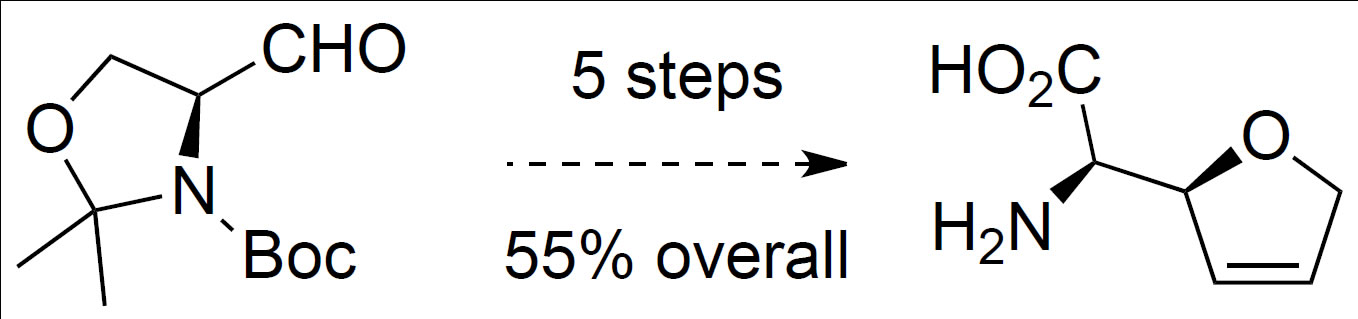
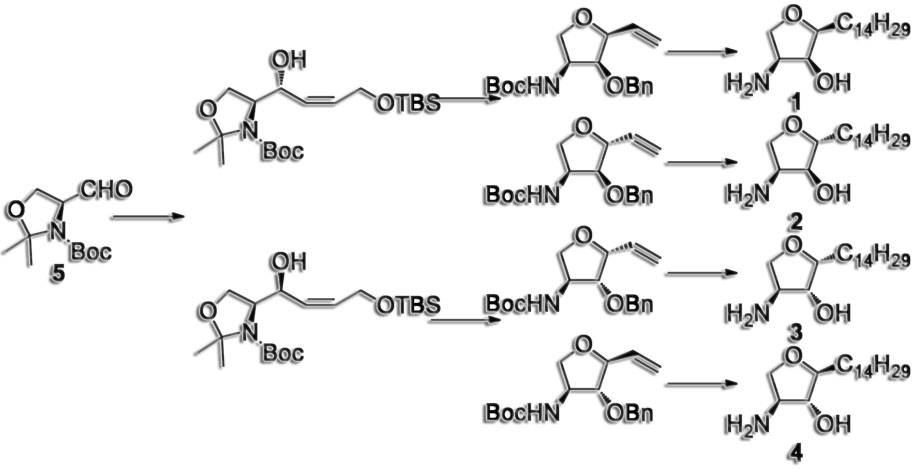
Passiniemi, M.; Koskinen, A.M.P. ‘Enantioselective synthesis of norfuranomycins.’ Tetrahedron Lett. 2011, 52, 6736–6738. ________________________________________________________________________________
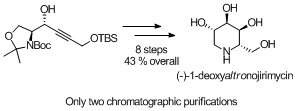
Koskinen, A.M.P. ‘Chirospecific Synthesis – Catalysis and Chiral Pool Hand in Hand.’ Pure Appl. Chem. 2011, 83, 435–443. ________________________________________________________________________________ Karjalainen, O.K.; Koskinen, A.M.P. ‘Rapid and practical synthesis of (-)-1-deoxyaltronojirimycin.’ Org. Biomol. Chem. 2011, 9, 1231–1236. ________________________________________________________________________________
Passiniemi, M.; Koskinen, A.M.P. ‘Enantioselective synthesis of Pachastrissamine (Jaspine B) and its diastereomers via h3-allylpalladium intermediates.’ Org. Biomol. Chem. 2011, 9, 1774–1783 ________________________________________________________________________________ Passiniemi, M.; Myllymäki, M.J.; Vuokko, J.; Koskinen, A.M.P. ’Demethylation of Aromatic Methyl Ethers Using Ionic Liquids under Microwave Irradiation.’ Lett. Org. Chem. 2011, 8, 48–52. ________________________________________________________________________________
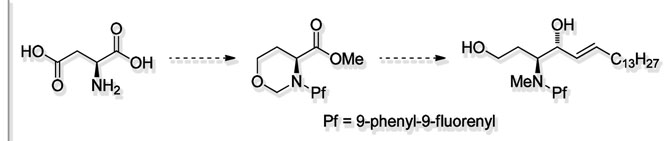
Karppanen, E.J.; Koskinen, A.M.P. ‘9-Phenyl-9-fluorenyl Group for Nitrogen Protection in Chirospecific Synthesis.’ Molecules 2010, 15, 6512-6547. ________________________________________________________________________________
Passiniemi, M.; Koskinen, A.M.P. ‘ A short and efficient synthesis of (2 S ,3 S ,4 S )- tert -butyl 3,4-dihydroxy-2-(methoxymethyl)-5-oxopyrrolidine-1-carboxylate.' Synthesis , 2010 , 2816-2822. ________________________________________________________________________________
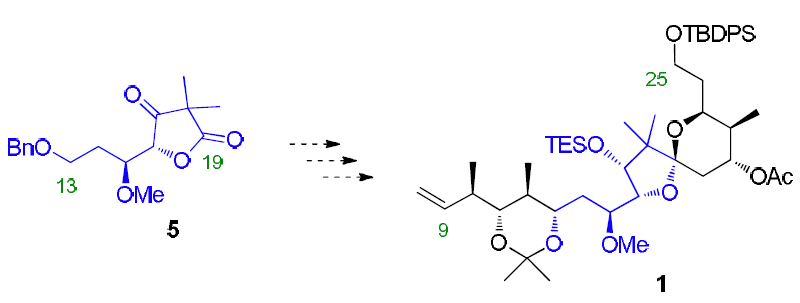
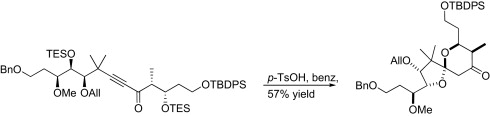
Habrant, D.; Koskinen, A.M.P. ‘ Towards the total synthesis of calyculin C: Preparation of the C 9 -C 25 spiroketal-dipropionate unit.' Org. Biomol. Chem. 2010 , DOI: 10.1039/C0OB00092B. ________________________________________________________________________________
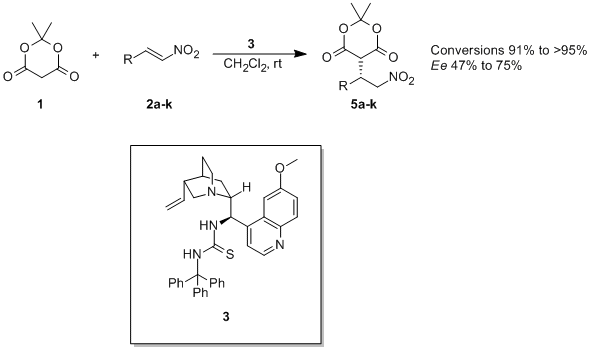
Kataja, A.O.; Koskinen, A.M.P. ‘Asymmetric organocatalytic Michael-addition of Meldrum's acid to nitroalkenes: probing the mechanism of bifunctional thiourea organocatalysts.' Arkivoc 2010 , (ii), 205-223. ________________________________________________________________________________
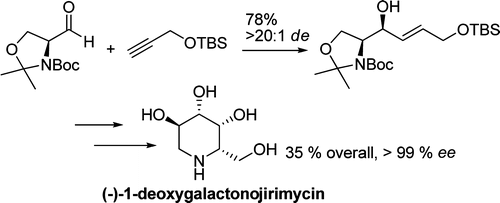
Habrant, D.; Vesa Rauhala, V.; Koskinen, A.M.P. ' Conversion of carbonyl compounds to alkynes: general overview and recent developments.' Chem. Soc. Rev. 2010 , 39 , 2007-2017. ________________________________________________________________________________ Karjalainen, O.K.; Passiniemi, M.; Koskinen, A.M.P. 'Short and efficient synthesis of l -deoxygalactonojirimycin.' Org. Lett. 2010 , 12 , 1145-1147. ________________________________________________________________________________ Calyculins, highly cytotoxic polyketides, originally isolated from the marine sponge Discodermia calyx by Fusetani and co-workers, belong to the lithistid sponges group. These molecules have become interesting targets for cell biologists and synthetic organic chemists. The serine/threonine protein phosphatases play an essential role in the cellular signalling, metabolism, and cell cycle control. Calyculins express potent protein phosphatase 1 and 2A inhibitory activity, and have therefore become valuable tools for cellular biologists studying intracellular processes and their control by reversible phosphorylation. Calyculins might also play an important role in the development of several diseases such as cancer, neurodegenerative diseases, and type 2- diabetes mellitus . The fascinating structures of calyculins have inspired various groups of synthetic organic chemists to develop total syntheses of the most abundant calyculins A and C. However, with fifteen chiral centres, a cyano-capped tetraene unit, a phosphate-bearing spiroketal, an anti , anti , anti dipropionate segment, an a-chiral oxazole, and a trihydroxylated ?-amino acid, calyculins reach versatility that only few natural products can surpass, and truly challenge modern chemists' asymmetric synthesis skills. Fagerholm, A.E.; Habrant, D.; Koskinen, A.M.P. ‘Calyculins and Related Marine Natural Products as Serine-Threonine Protein Phosphatase PP1 and PP2A Inhibitors and Total Syntheses of Calyculin A, B, and C.' Marine Drugs 2010, 8, 122-172. ________________________________________________________________________________
Koivisto, J.J.; Kumpulainen, E.T.T.; Koskinen, A.M.P. ‘Conformational Ensembles of Flexible b -Turn Mimetics in DMSO- d6.' Org. Biomol. Chem. 2010, 8, 2103-2116. ________________________________________________________________________________
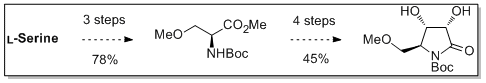
Sauerland, S.J.K.; Castillo-Meléndez, J.A.; Nättinen, K.; Rissanen, K.; Koskinen, and A.M.P. ‘Enantioselective synthesis of homo-sphingosine derivatives from L-aspartic acid.’ Synthesis 2010, 757-763 . ________________________________________________________________________________
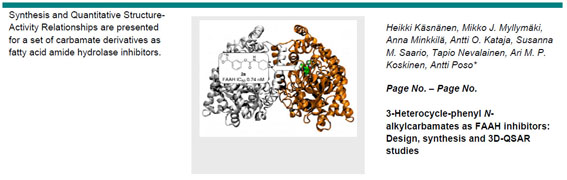
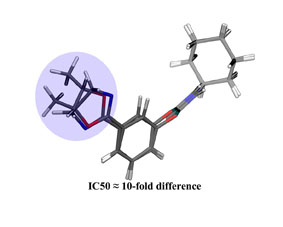
Käsnänen, H.; Myllymäki, M.J.; Minkkilä, A.; Kataja, A.O.; Saario, S.M.; Nevalainen, T.; Koskinen, A.M.P.; Poso, A. ’ 3-Heterocycle-phenyl N-alkylcarbamates as FAAH inhibitors: Design, synthesis and 3D-QSAR studies.’ Chem.Med.Chem. 2010 , 5 , 213-231. ________________________________________________________________________________
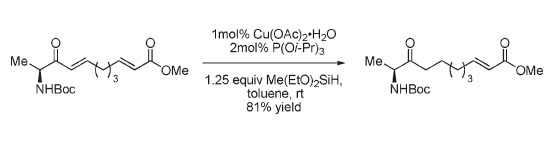
Pelšs, A.; Kumpulainen, E.T.T.; Koskinen, A.M.P. ‘Highly Chemoselective Copper Catalyzed Conjugate Reduction of Stereochemically Labile α,β-Unsaturated Amino Ketones.’ J. Org. Chem. 2009, 74, 7598-7601. ________________________________________________________________________________
Kallatsa, O.A.; Nissinen, M.; Koskinen, A.M.P. ‘Polyhydroxylated indolizidine alkaloids - synthesis of dideoxycastanospermine.’ Tetrahedron 2009, 65, 9285-9290. ________________________________________________________________________________
Habrant, D.; Stewart, A.J.W.; Koskinen, A.M.P. ‘Towards the total synthesis of Calyculin C: preparation of the C13-C25 spirocyclic core.’ Tetrahedron 2009, 65, 7927-7934. ________________________________________________________________________________
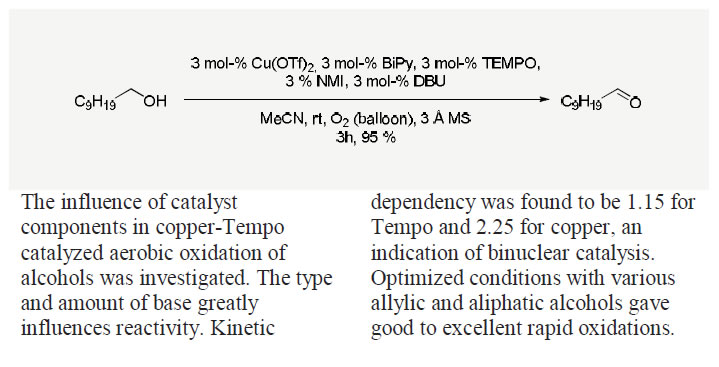
Kumpulainen, E.T.T.; Koskinen, A.M.P. ‘Catalytic Activity Dependency on Catalyst Components in Aerobic Copper-TEMPO Oxidation.’ Chem. Eur. J. 2009, 15, 10901-10911. ________________________________________________________________________________
Myllymäki, M.J.; Käsnänen, H.; Kataja, A.O.; Lahtela-Kakkonen, M.; Saario, S.M.; Poso, A.; Koskinen, A.M.P. ‘Chiral 3-(4,5-dihydrooxazol-2-yl)phenyl alkylcarbamates as novel FAAH inhibitors: insight into FAAH enantioselectivity by molecular docking and interaction fields.’ Eur. J. Med. Chem. 2009, 44, 4179-4191. ________________________________________________________________________________
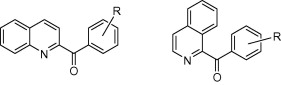
Reux, B.; Nevalainen, T.; Koskinen, A.M.P. ’ Synthesis and agonist properties of novel quinoline and isoquinoline derivatives toward the cannabinoid receptor CB2.’ Bioorg. Med. Chem. 2009, 17, 4441-4447. ________________________________________________________________________________
Minkkilä, A.; Myllymäki, M.; Saario, S.M.; Castillo-Melendez, J.A.; Koskinen, A.M.P.; Fowler, C.J.; Leppänen, J.; Nevalainen, T. ‘The Synthesis and Biological Evaluation of para-Substituted Phenolic N-Alkyl Carbamates as Endocannabinoid Hydrolyzing Enzyme Inhibitors.’ Eur. J. Med. Chem. 2009, 44, 2994-3008. ________________________________________________________________________________
Bassas, O.; Huuskonen, J.; Rissanen, K.; Koskinen, A.M.P. ’A Simple Organocatalytic Enantioselective Synthesis of Pregabalin.’ Eur. J. Org. Chem. 2009, 1340-1351. ________________________________________________________________________________
|